The Future of Energy Storage: Exploring Next-Generation Lithium Batteries
In the quest for sustainable energy solutions, advanced battery technettologies are at the forefront of innovation. As the world transitions to renewable energy and electric mobility, the demand for high-performance, cost-effective, and safe energy storage systems has never been greater. While lithium-ion batteries have powered our devices and vehicles for decades, their limitations in energy density, cost, and safety have spurred research into next-generation alternatives. Among these, Lithium-Sulfur (Li-S) batteries, Solid-State batteries, and Sodium-Ion batteries stand out as promising candidates, each offering unique advantages and facing specific challenges. Additionally, emerging technologies like Lithium-Air batteries hint at even greater possibilities. This article explores these cutting-edge technologies, their potential to revolutionize energy storage, and the hurdles they must overcome to power the future.

Lithium-Sulfur (Li-S) Batteries
What Are Li-S Batteries?
Lithium-Sulfur (Li-S) batteries are a promising advancement in battery technology, leveraging a lithium metal anode and a sulfur cathode. Unlike conventional lithium-ion batteries that use metal oxide cathodes, Li-S batteries operate by having lithium ions migrate from the anode to the cathode during discharge, reacting with sulfur to form lithium polysulfides and ultimately lithium sulfide (Li2S). This chemistry enables Li-S batteries to achieve a theoretical energy density of up to 2600 Wh/kg, significantly higher than the 250-300 Wh/kg of current lithium-ion batteries.
Advantages
- High Energy Density: The standout feature of Li-S batteries is their potential to store significantly more energy per unit weight, making them ideal for weight-sensitive applications like electric vehicles (EVs) and aerospace. For example, they could enable electric aircraft for short-range flights or vertical take-off and landing by 2050 [1].
- Cost-Effectiveness: Sulfur is abundant and inexpensive compared to cobalt and nickel used in lithium-ion batteries, potentially reducing production costs.
- Environmental Benefits: Sulfur is more environmentally friendly, and using lithium metal anodes reduces reliance on rare earth metals, aligning with sustainability goals.
Disadvantages
- Cycle Life: Li-S batteries suffer from shorter cycle lives due to the polysulfide shuttle effect, where polysulfides dissolve into the electrolyte, causing capacity fade over time.
- Volume Expansion: Sulfur undergoes significant volume changes during cycling, leading to mechanical stress and degradation of the battery structure.
- Low Conductivity: Sulfur’s low electrical conductivity (5×10⁻³⁰ S⋅cm⁻¹ at 25°C) requires conductive additives like carbon, adding weight and complexity [2].
Current Status and Developments
Recent research has made strides in addressing these challenges. For instance, a study from Argonne National Laboratory introduced a redox-active interlayer that reduces polysulfide shuttling, achieving high capacity retention over 700 charge-discharge cycles [3]. Companies like Sion Power have developed Li-S batteries with practical energy densities of around 350 Wh/kg, though still below theoretical limits. Ongoing efforts focus on improving electrolytes and cathode materials to enhance cycle life and conductivity, with potential applications in drones, satellites, and EVs.
| Aspect | Details |
|---|---|
| Energy Density | Theoretical: 2600 Wh/kg; Practical: ~350 Wh/kg |
| Cost | Lower due to abundant sulfur |
| Cycle Life | Limited by polysulfide shuttle; improving with new interlayers |
| Applications | EVs, aerospace, drones |
Solid-State Batteries
What Are Solid-State Batteries?
Solid-State batteries replace the liquid electrolyte found in traditional lithium-ion batteries with a solid electrolyte, often paired with a lithium metal anode. This design enhances safety and performance, making them a leading candidate for next-generation lithium batteries. The solid electrolyte can be made from materials like oxides, sulfides, or polymers, each with unique properties.
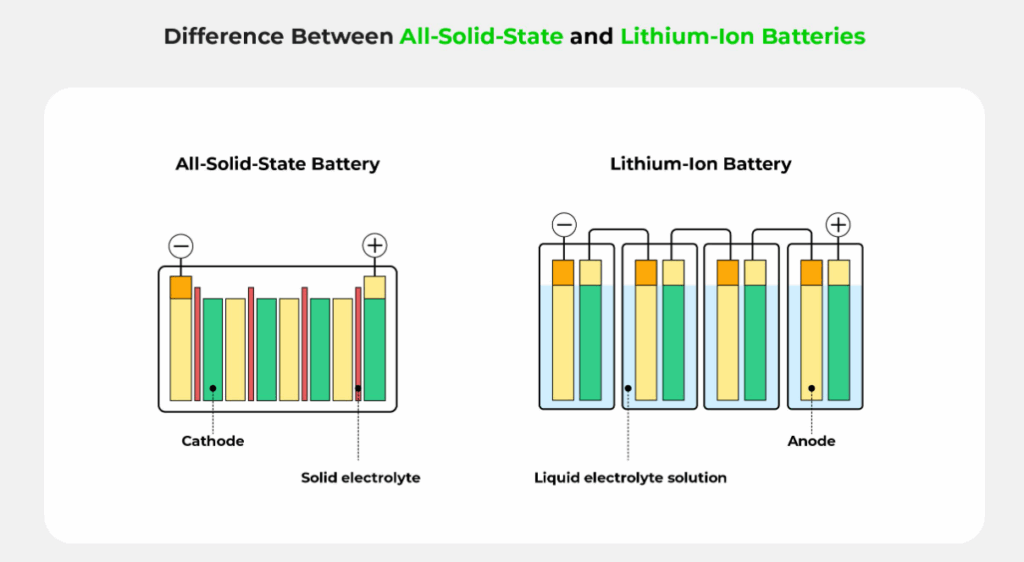
Advantages
- Enhanced Safety: Solid electrolytes are non-flammable, significantly reducing the risk of fires and thermal runaway compared to liquid electrolytes [4].
- Higher Energy Density: Lithium metal anodes enable higher energy storage, potentially exceeding lithium-ion batteries’ capacity.
- Longer Lifespan: Reduced side reactions and dendrite formation can lead to longer cycle lives.
- Faster Charging: Some solid-state designs allow faster ion transport, enabling rapid charging, as demonstrated by Panasonic’s prototype charging from 10% to 80% in 3 minutes [5].
- Wide Temperature Range: Solid-state batteries can operate effectively in extreme temperatures, from -50°C to 125°C [6].
Disadvantages
- Manufacturing Complexity: Producing solid electrolytes and ensuring good electrode-electrolyte contact is challenging and costly.
- Material Limitations: Finding solid electrolytes with high ionic conductivity at room temperature remains a hurdle.
- Scalability: Most developments are at the laboratory stage, with mass production facing engineering challenges.
- Dendrite Formation: While safer, lithium dendrite growth can still cause short circuits in some designs.
Current Status and Developments
Companies like Toyota and QuantumScape are leading the charge, with Toyota aiming to commercialize solid-state batteries for EVs by the late 2020s. QuantumScape has reported prototypes with high energy density and fast charging capabilities. Research focuses on improving solid electrolytes and interfacial engineering to suppress dendrite formation [4]. For example, Maxell has begun producing solid-state batteries for industrial machinery, and Yoshino introduced solid-state portable solar generators in 2023 [5].
| Aspect | Details |
|---|---|
| Energy Density | Potentially 2.5x higher than lithium-ion |
| Safety | Non-flammable electrolytes |
| Cycle Life | Improved, but depends on material stability |
| Applications | EVs, consumer electronics, industrial machinery |
Sodium-Ion Batteries
What Are Sodium-Ion Batteries?
Sodium-Ion batteries operate similarly to lithium-ion batteries but use sodium ions (Na⁺) as charge carriers. They employ different cathode and anode materials, such as sodium layered transition metal oxides or Prussian white, and do not rely on lithium. Their similarity to lithium-ion technology allows for integration into existing manufacturing processes.
Advantages
- Abundant Resources: Sodium is far more abundant than lithium, primarily sourced from saltwater, making it a sustainable option [7].
- Cost-Effectiveness: Materials like iron-based cathodes are cheaper, with sodium-ion batteries costing about 30% less than lithium-ion batteries [8].
- Safety: Sodium-ion batteries exhibit greater thermal stability, reducing fire risks and eliminating dendrite-related issues.
- Manufacturing Compatibility: They can be produced using similar processes to lithium-ion batteries, requiring only minor adjustments to existing facilities.
Disadvantages
- Lower Energy Density: Current sodium-ion batteries offer 140-160 Wh/kg, compared to 180-250 Wh/kg for lithium-ion batteries [7].
- Shorter Cycle Life: Sodium’s larger ionic radius causes mechanical stress, leading to faster degradation, though some designs achieve up to 4000 cycles [9].
- Size and Weight: Lower energy density results in bulkier batteries, limiting their use in compact applications.
Current Status and Developments
Sodium-ion batteries are gaining traction for grid storage and low-cost EVs. CATL launched a first-generation sodium-ion battery with 160 Wh/kg in 2021, and companies like Faradion and Tiamat are advancing commercialization [7]. Applications include stationary energy storage for renewable energy and low-speed electric vehicles, such as scooters by Yadea. Research aims to improve energy density and cycle life, with projections suggesting sodium-ion batteries could capture 23% of the stationary storage market by 2030 [9].
| Aspect | Details |
|---|---|
| Energy Density | 140-160 Wh/kg, expected to improve |
| Cost | ~30% lower than lithium-ion |
| Cycle Life | Up to 4000 cycles in some designs |
| Applications | Grid storage, low-cost EVs |
Other Emerging Technologies
Beyond Li-S, Solid-State, and Sodium-Ion batteries, other innovative technologies are on the horizon. Lithium-Air (Li-Air) batteries, for instance, offer a theoretical energy density of up to 5210 Wh/kg, comparable to gasoline, by combining lithium with oxygen from the air to form lithium peroxide [10]. This could enable EVs with ranges exceeding 500 km on a single charge. However, challenges like low efficiency, short cycle life, and sensitivity to moisture and CO2 limit their current viability. Research, such as that at Argonne National Laboratory, is exploring solid electrolytes to improve performance [11].
Other metal-ion batteries, such as those using magnesium or aluminum, are also under investigation. These could offer higher energy densities and lower costs but are in early research stages, with commercialization further off.
Conclusion
The landscape of battery technology is evolving rapidly, with Lithium-Sulfur, Solid-State, and Sodium-Ion batteries leading the charge toward more efficient, safe, and sustainable energy storage. Li-S batteries promise high energy density for lightweight applications, Solid-State batteries offer safety and performance for EVs, and Sodium-Ion batteries provide a cost-effective alternative for grid storage. While challenges like cycle life, scalability, and material limitations persist, ongoing research and investments from companies like Toyota, QuantumScape, and CATL are paving the way for commercialization. Emerging technologies like Lithium-Air batteries hint at even greater possibilities, potentially transforming industries from aviation to renewable energy. As these technologies mature, they hold the potential to make renewable energy more accessible and electric transportation more efficient and affordable, driving us toward a cleaner, more sustainable future.
Citations
- Faraday Institution – Lithium-Sulfur Batteries
- Wikipedia – Lithium-Sulfur Battery
- Argonne National Laboratory – Lithium-Sulfur Batteries
- ScienceDirect – A comprehensive review of solid-state batteries
- Wikipedia – Solid-state battery
- Saft – Three battery technologies
- Wikipedia – Sodium-ion battery
- evlithium – Sodium-ion Battery Advantages and Disadvantages
- Iberdrola – Sodium-ion batteries
- Wikipedia – Lithium-air battery
- Argonne National Laboratory – Lithium-Air Battery
